Home
A comprehensive resource for safe and responsible laser use
US: UPDATED - FDA wants to allow only red laser pointers, calling all other colors "defective"

FDA’s primary concern is green lasers’ interference with the vision of vehicle operators including pilots. Green lasers are involved in over 90% of incidents where pilots reported to the Federal Aviation Administration (FAA) that they saw or were illuminated by laser light during a flight. (The charts below were added in January 2017 after the 2016 FAA final numbers came out.)
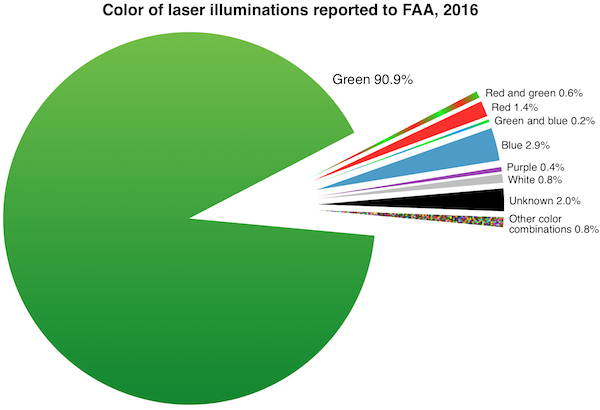
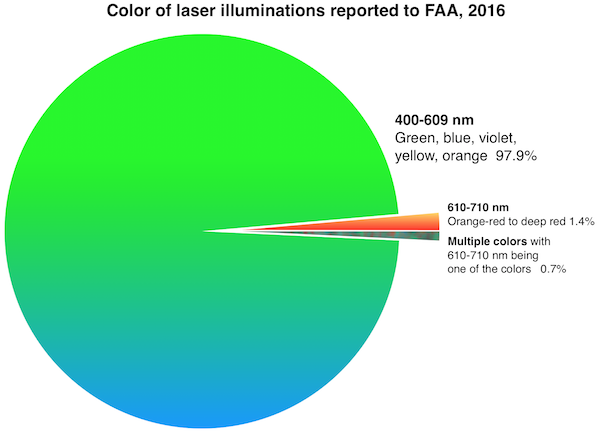
FDA is also worried about blue lasers which can have greater visibility to night-adapted eyes than red lasers of equivalent power.
Thus FDA is applying the “defective” label — giving them added authority over potentially injurious products — because of what they consider to be a well-known, established public safety hazard to operators of vehicles, aircraft and watercraft.
What FDA is trying to accomplish
FDA has two main goals:
1) “Turn back the clock” to the 1990s and early 2000s when almost all laser pointers were red. According to the agency, red light has the least interference with pilot vision, compared to equivalent-power green beams which can appear up to 28 times brighter. During this period there were dozens or low hundreds of reported laser/aviation incidents per year, compared with 7,703 incidents in 2015 and 7,442 incidents in 2016.
2) Make it much easier for customs and law enforcement to identify illegal laser pointers simply by their color. Red and orange-red laser pointers would be permitted; all others would be prohibited for general sales.
In addition, FDA sought to address requests from legislators including Senator Chuck Schumer (D-NY). After high-profile incidents, lawmakers have written to FDA, asking for a ban on green pointers due to their vision-blocking abilities being a risk to pilots and passengers.
Who would be affected
FDA’s proposed color-based prohibition would only affect the manufacture, importation and sales of laser pointer products introduced into commerce. Although pointers fall under the FDA’s “surveying, leveling and alignment” (SLA) control, only pointers as defined by FDA would be restricted to red. Standard SLA equipment would not be affected — they could use any color beam.
Individuals such as hobbyists who manufacture their own laser products for their personal use would be free from FDA laser product regulations. This is because such individuals would not be considered manufacturers by FDA.
Since federal law cannot control individual use or misuse, states and localities could impose their own regulations. (A few states and localities already have their own restrictions on use and/or possession; these are not currently based on the color of the laser.) One benefit of FDA’s proposal is that any new state and local laws could “piggyback” on FDA’s color-based restrictions. That would make it easier for local law enforcement to use color to easily identify whether a person possessed a prohibited or permitted laser.
FDA’s proposal and rationale was stated in draft amendments presented October 25 to an FDA advisory panel known as “TEPRSSC”.
Click to read more...